Abstract
Introduction: Multiple studies in industrialized countries indicate that postpartum anemia affects 20-50% of patients immediately after delivery (Barroso F et al Eur J Obstet Gynecol Reprod Biol 2011; Bergmann RL et al Eur J Obstet Gynecol Reprod Biol 2010; Urquizu IBX et al Med Clin (Barc) 2016). A growing body of literature suggests that intravenous (IV) iron improves time to anemia recovery in postpartum patients (Breymann C et al. Int J Gynaecol Obstet. 2008; Seid MH et al, Am J Obstet Gynecol 2008). We therefore developed recommendations for the use of IV iron on our Labor and Delivery (L&D) service to treat postpartum anemia. Three months after initiation of the IV iron recommendations, we performed an audit to assess its use.
Methods: The UCSF pharmacy identified medical record numbers for obstetrical patients given IV iron sucrose 5/1/17 - 10/31/17, which spans 3 months pre-release of the IV iron protocol through the first 3 months after protocol release. Seventy-one patients were identified and their charts were individually reviewed to determine the dose and number of IV iron infusions received, interval between infusions when more than one infusion was given, the IV location for each infusion, and whether phlebitis was reported. Phlebitis was considered to have occurred if a physician or nursing note indicated that the patient had pain, swelling or erythema in an arm correlating to documented site of IV iron infusion. This study was conducted under UCSF Institutional Review Board approval number 17-22831.
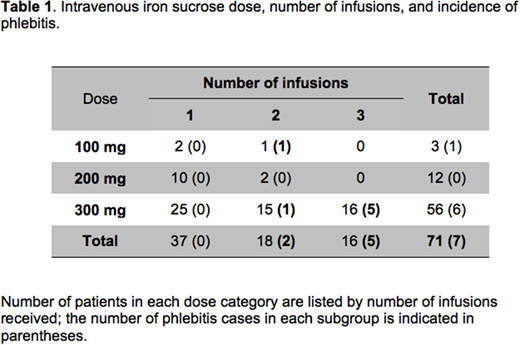
Results: Patients received 100 mg, 200 mg, or 300 mg of iron sucrose per infusion. Each infusion was administered over 30-90 minutes and patients received 1, 2, or 3 IV iron infusions. Venofer® is the only IV iron sucrose product received by patients in this study. The overall incidence of phlebitis was 9.9% (7 of 71 patients), higher than expected based on the previously published safety data and the published experience using IV iron sucrose in the postpartum setting (Breymann C et al. Eur J Clin Invest. 2000; Westad S et al. Acta Obstet Gynecol Scand. 2008). In addition, we found an increase in phlebitis incidence with increasing number of IV infusions: phlebitis was seen in 2 of 18 (11%) patients who received 2 iron infusions on consecutive days via a single IV site, while patients who received 300 mg iron sucrose on 3 consecutive days (5 out of 16 patients; Table 1) had a 31% incidence of phlebitis. Importantly, the phlebitis incidence was 50% for the 10 patients who received 3 infusions of 300 mg iron sucrose at a single IV site. Interestingly, in 6 patients who received 3 infusions of 300 mg iron sucrose on 3 consecutive days but had their peripheral IV re-sited between infusions, none developed phlebitis. No phlebitis was reported in 37 patients who received a single infusion of iron sucrose (100 - 300 mg dose range). Of the 7 phlebitis cases, 3 patients were readmitted to the hospital for evaluation and treatment. Three patients returned to our OB clinic or to L&D Triage for evaluation. One patient communicated her symptoms by telephone encounter.
Discussion. IV iron safety analyses have historically focused on dose limitations and the risk for infusion reaction and anaphylaxis. We identified an increasing incidence of post-infusion phlebitis with increasing number of infusions of daily iron sucrose in patients admitted to our L&D service, an adverse event not previously highlighted in published safety literature for iron sucrose. We found the phlebitis events to be clinically significant for the patient, and to result in increased healthcare utilization post-infusion. It is our opinion that this adverse effect deserves attention because of the patient discomfort, inconvenience, and increased healthcare resource utilization. Physicians treating patients for iron-deficiency anemia, including hospitalists, hematologist, and OB-Gyn providers, should be aware of post-infusion phlebitis as a possible complication of iron sucrose therapy. Based on our findings, we recommend against daily IV iron sucrose infusions through a single peripheral venous access site. In addition, other formulations that allow for complete iron replacement in a single infusion session offer a convenience advantage to doctor and patient and may minimize the risk of phlebitis we observed with serial daily iron infusions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal